Nitric oxide and bromine were allowed to react in a sealed container. When equilibrium was reached PNO = 0.526 atm, 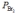 = 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction.
= 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction.
2NO(g) + Br2(g)  2NOBr(g)
2NOBr(g)
Definitions:
Structural-Functionalists
Sociologists who focus on understanding society as a complex system, where its components work together to maintain cohesion and order.
Labor Theory of Value
An economic theory, most closely associated with Karl Marx, which posits that the value of a commodity is determined by the socially necessary labor time required for its production.
Exchange Value
The worth of a good or service determined by what it can be traded or exchanged for in the market.
Use Value
The utility of a good or service for satisfying a need or providing a benefit to the user, as opposed to its exchange value or price.
Q11: Predict the products for the reaction of
Q18: What is the pH of a 0.0125
Q19: In hydrogen iodide _ are the most
Q20: Which, if any, of the following aqueous
Q32: When an alkali metal combines with a
Q35: What is the value of the equilibrium
Q59: Potassium is a strong reducing agent.
Q67: A red glaze on porcelain can be
Q91: Which of the following aqueous systems has
Q92: Butyric acid is responsible for the odor