Calculate the vapor pressure of a solution prepared by dissolving 0.500 mol of a non-volatile solute in 275 g of hexane ( 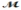 = 86.18 g/mol) at 49.6°C. P°hexane = 400.0 torr at 49.6°C.
= 86.18 g/mol) at 49.6°C. P°hexane = 400.0 torr at 49.6°C.
Definitions:
Presidential Election
The process by which the president of the United States is selected, involving nationwide votes and the Electoral College system.
Southern Republicans
Republicans residing in the Southern United States, historically significant for their role during the Reconstruction era and their political evolution over time.
Territorial Government
A form of governmental authority that operates in a geographically defined territory, typically not yet a full-fledged state within a union.
Suffrage
The right to vote in public elections, a fundamental principle of democratic societies.
Q5: The reaction of nitric oxide to form
Q12: The vapor pressure of pure acetone (propanone)
Q38: Which one of the following sets of
Q51: A salesperson who telecommutes rather than travel
Q78: The ammonium ion, NH<sub>4</sub><sup>+</sup>, is a weak
Q98: According to VSEPR theory, a molecule with
Q99: When elements from a group exhibit more
Q104: Acetaminophen is a widely used and an
Q111: Which of the following is a relatively
Q149: Which of the following approaches to selling