Calculate the freezing point of a solution made by dissolving 3.50 g of potassium chloride ( 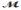 = 74.55 g/mol) in 100.0 g of water. Assume ideal behavior for the solution; Kf = 1.86°C/m.
= 74.55 g/mol) in 100.0 g of water. Assume ideal behavior for the solution; Kf = 1.86°C/m.
Definitions:
Major Inventory
Major Inventory refers to the significant stock of goods a company holds for the purpose of sale or production in its normal business operations.
Valuation
The process of determining the current worth of an asset or a company, based on metrics such as earnings, sales, assets, and more.
Unit Cost
The cost incurred to produce, store, and sell one unit of a product or service.
Unit Price
The cost assigned to a single unit of a product or service.
Q18: The area of a 15-inch pizza is
Q20: The equilibrium constant Kc for the reaction
Q25: According to molecular orbital (MO) theory, the
Q29: The network of physical things, vehicles, devices,
Q37: Today's professional salesperson is more likely to
Q47: At a pressure of one billionth (10<sup>-9</sup>)
Q64: Colligative properties depend on<br>A) the chemical properties
Q68: Which compound, if any, will be optically
Q95: A type of m-commerce that simply involves
Q96: Consider the molecular substances I<sub>2</sub>, H<sub>2</sub>O, and