Which of the following statements describes the correct method of preparation of 1.00 L of a 2.0 M urea solution? 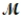 urea = 60.06 g/mol
urea = 60.06 g/mol
Definitions:
Progression Principle
In fitness and training, the idea that the intensity and duration of exercise should increase gradually over time for optimal improvement.
Need for Power
An individual's desire to influence, control, or have authority over others.
Social Power
The ability of an individual or group to influence or control others within a social or organizational context.
Personal Gratification
The feeling of pleasure or satisfaction that comes from achieving something, enjoying an activity, or fulfilling a desire.
Q21: A dictionary has the following definition for
Q25: The formal charge on Cl in the
Q28: Which one of the following substances does
Q31: Predict the products for the following set
Q36: The nitrate anion is<br>A) a strong acid.<br>B)
Q47: The heat of solution is the total
Q69: Dacron is a _ compound and is
Q70: The mass of a sample is 550
Q72: Which of the following would you predict
Q89: Which of the following is likely to