Calculate the vapor pressure of a solution prepared by dissolving 0.500 mol of a non-volatile solute in 275 g of hexane ( 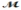 = 86.18 g/mol) at 49.6°C. P°hexane = 400.0 torr at 49.6°C.
= 86.18 g/mol) at 49.6°C. P°hexane = 400.0 torr at 49.6°C.
Definitions:
Malthus
Refers to Thomas Robert Malthus, an English economist and demographer known for his theories on population growth and its impacts on resources.
European Development
The process of economic, social, and political change and growth in European countries, often involving integration, innovation, and the handling of challenges such as migration and regional disparities.
One-child Policy
A birth control policy formerly implemented by China, aimed at controlling the population by limiting families to having only one child.
Spiralling Population
A scenario where a population grows rapidly due to a combination of high birth rates and lower mortality rates, often resulting in increased stress on resources and infrastructure.
Q8: Electrolyte solutions generally behave less ideally as
Q11: The strongest intermolecular interactions between ethyl alcohol
Q22: Which one of the following statements about
Q41: The appropriate number of significant figures in
Q56: Bond angles of 180° only occur around
Q59: Identify the principal organic products for the
Q60: The phase diagram of a substance shows
Q79: Ethane (C<sub>2</sub>H<sub>6</sub>) is much more reactive than
Q92: What is the mass-action expression, p, for
Q140: A traditional advertising medium can be used