Calculate the freezing point of a solution made by dissolving 3.50 g of potassium chloride ( 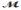 = 74.55 g/mol) in 100.0 g of water. Assume ideal behavior for the solution; Kf = 1.86°C/m.
= 74.55 g/mol) in 100.0 g of water. Assume ideal behavior for the solution; Kf = 1.86°C/m.
Definitions:
Cost of Goods Sold
The direct costs attributable to the production of the goods sold by a company.
Return on Equity Ratio
A measure of financial performance calculated by dividing net income by shareholders' equity, indicating how well a company uses investments to generate earnings growth.
Quality of Income Ratio
A financial metric that evaluates the ability of a company to translate its earnings into cash, reflecting the quality and sustainability of its earnings.
Cash Ratio
A liquidity metric that measures a company's ability to cover its short-term liabilities with its cash and cash equivalents.
Q16: For the reaction A(g) + 2B(g) →
Q22: Which of the following is an accurate
Q30: Aspirin is an effective and widely used
Q34: Which of the following atoms has the
Q35: Hexachlorophene is used as a disinfectant in
Q79: Gases with high boiling points tend to
Q86: What type of software do salespeople use
Q86: Which of the following pairs is arranged
Q97: Predict the products for the following set
Q135: The FTC regulates the opt-out list for