Liquid ammonia (boiling point = -33.4°C) can be used as a refrigerant and heat transfer fluid. How much energy is needed to heat 25.0 g of NH3(l) from -65.0°C to -12.0°C?
Specific heat capacity, NH3(l) : 4.7 J/(g·K)
Specific heat capacity, NH3(g) : 2.2 J/(g·K)
Heat of vaporization: 23.5 kJ/mol
Molar mass, 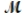 : 17.0 g/mol
: 17.0 g/mol
Definitions:
Emotion Regulation
the process by which individuals influence which emotions they have, when they have them, and how they experience and express these emotions.
Cognitive Regulation
The ability to control one's thought processes, manage cognitive activities, and adaptively apply mental skills to various situations.
Etic Approach
An approach that emphasizes the universal aspects of human behavior and development.
Emic Approach
An approach that explores how behavior and development take place within specific cultural contexts.
Q10: Dimethylglyoxime, DMG, is an organic compound used
Q13: Liquid sodium can be used as a
Q19: What is the molecular shape of NO<sub>2</sub><sup>-</sup>
Q28: Which of the following pairs of ions
Q31: Predict the products for the following set
Q43: Which of the following is not an
Q46: Predict the products for the following set
Q46: A sample of octane in equilibrium with
Q61: A detailed explanation of natural phenomena that
Q68: Certain Period 2 elements exhibit behaviors similar