In the following Lewis structure for phosphate, phosphorus has a formal charge of ____ and an oxidation number of ____. 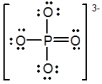
Definitions:
Six-Figure Accuracy
Precision to the level of one part in a hundred thousand, often used in the context of scientific measurements or financial calculations.
Calculate
Determine mathematically.
Percent Change
The extent to which a quantity has increased or decreased, expressed as a percentage.
Initial Value
The starting monetary or numerical value of an investment, account, or another financial instrument before any earnings or losses.
Q4: Which of the following correctly shows how
Q12: The difference between a student's experimental measurement
Q17: The Clausius-Clapeyron equation is used in calculations
Q45: Coca-Cola's Minute Maid unit is introducing a
Q59: In a reversible reaction, a catalyst will
Q67: In which step of the sales process
Q76: Sodium hypochlorite is used<br>A) in chemical analysis.<br>B)
Q95: How many resonance structures are possible for
Q118: The wheel-of-retailing hypothesis can be used to
Q134: A young couple contacted a realtor about