Calculate the mass defect for an atom of 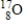 whose isotope mass is 16.9991 g/mol. mass of proton (
whose isotope mass is 16.9991 g/mol. mass of proton ( 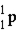 )
)
1) 00783 g/mol
Mass of neutron ( 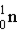 )
)
1) 00867 g/mol
Definitions:
Annoying Sound
A noise that is irritating or unpleasant to listen to.
Seatbelt
A safety device fitted in vehicles, designed to secure the occupant against harmful movement that may result from sudden stops or impacts.
Skinner Box
Is a controlled environment used to observe and study the behavior of animals, especially in experiments involving operant conditioning.
Variable Ratio Schedule
A reinforcement schedule in operant conditioning where rewards are provided after an unpredictable number of responses, promoting high and steady rates of behavior.
Q8: Consumers looking for lasting values and tangible
Q10: Converting existing chain outlets into franchises is
Q10: Which is not a characteristic of market
Q11: How many donor atoms are present in
Q11: _ consists of the middle point of
Q12: Tax planning will address the issue of:<br>A)
Q28: Write the symbol for calcium._
Q50: Catalytic steam reformation of hydrocarbons (such as
Q62: An element's most stable ion forms an
Q175: All isotopes with atomic number greater than