Calculate the mass defect for an atom of 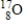 whose isotope mass is 16.9991 g/mol. mass of proton (
whose isotope mass is 16.9991 g/mol. mass of proton ( 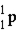 )
)
1) 00783 g/mol
Mass of neutron ( 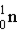 )
)
1) 00867 g/mol
Definitions:
Canned Surveys
Pre-packaged surveys designed for general use across a variety of contexts without modification to the questions.
Difficult To Administer
Describes processes, systems, or regulations that are complex or cumbersome to manage, often due to intricacy, multitude of components, or lack of clarity.
Farsighted Organizations
Organizations that prioritize long-term goals and outcomes over immediate gains, often incorporating strategic planning and forward-thinking.
Strategic Farsightedness
The ability of an organization to look beyond immediate challenges and plan for long-term opportunities and risks.
Q3: Marketing activities:<br>A) are always overlooked by entrepreneurs.<br>B)
Q9: The more precise scales contain all the
Q17: Inventory turnover refers to:<br>A) customer handling of
Q17: Beyond _,the neutron-to-proton ratio is always greater
Q17: The largest renewable source of electricity production
Q24: A franchise system can best be characterized
Q72: What is the name of the following
Q81: Use your knowledge of group properties to
Q87: Which particle does the nuclide symbol <img
Q123: What percentage of the world's electricity is