In an octahedral complex,electrons in  and
and 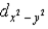 orbitals experience a greater repulsion from the lone pairs of electrons on ligands because these orbitals
orbitals experience a greater repulsion from the lone pairs of electrons on ligands because these orbitals
Definitions:
Instructor's Role
The set of responsibilities and duties an instructor has to facilitate learning among students, including preparing lessons, delivering lectures, and assessing student progress.
Syllabus
An academic document that outlines the structure, content, and evaluation methods of a course.
Instructor's Expectations
Instructor's expectations are the standards and requirements that an educator has for their students' performance and behavior in a course.
Adjuncts
Part-time faculty members hired by educational institutions often to teach specific courses.
Q8: Which of the following is the first
Q10: What is the oxidation state of each
Q17: What is the formula for the pentaamminehydroxochromium(III)ion?<br>A)
Q21: Offers of employment should include:<br>A) terms and
Q29: The following electrochemical cell has a potential
Q29: What is the pH of a 0.046
Q39: Aluminum(III)ion (Al<sup>3+</sup>)is reduced to solid aluminum at
Q49: What is the pH of a solution
Q63: Which of the following is the correct
Q80: When a Lewis acid and a Lewis