In an octahedral complex,electrons in  and
and 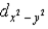 orbitals experience a greater repulsion from the lone pairs of electrons on ligands because these orbitals
orbitals experience a greater repulsion from the lone pairs of electrons on ligands because these orbitals
Definitions:
Federal Reserve
The central bank of the United States, responsible for setting monetary policy, supervising and regulating banks, and ensuring the stability of the financial system.
Required Reserve Ratio
The fraction of deposits that a bank is required to hold in reserve and not lend out, as mandated by a central banking authority.
Excess Reserves
The reserves that banks hold in excess of the required minimum, often held in the central bank, which can lend them out to generate interest.
New Loans
Any sums of borrowed money that have recently been provided by a lender to a borrower.
Q17: What is the formula for the pentaamminehydroxochromium(III)ion?<br>A)
Q37: The following has a potential of 0.34
Q38: The carbon-carbon bonding of cyclopropane is shown
Q42: A solution containing 10.mmol of CO<sub>3</sub><sup>2</sup><sup>−</sup>and 5.0
Q50: The majority of electricity generated in the
Q64: What is the H<sub>3</sub>O<sup>+</sup> concentration in 0.0072
Q70: The hydroxide ion concentration of a saturated
Q78: Which list contains the three most abundant
Q82: A 4.50 mg sample of a newly
Q146: Which of the following particles has the