The electrochemical reaction that powers a lead-acid storage battery is as follows: Pb(s) + PbO2(s) + 4 H+(aq) + 2 SO42-(aq) → 2 PbSO4(s) + 2 H2O( 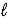 )
)
A single cell of this battery consists of a Pb electrode and a PbO2 electrode,each submerged in sulfuric acid.Which of the following reactions occurs at the cathode during discharge?
Definitions:
Standard Deviation
A quantification of the extent to which a group of numbers deviates from their average.
Process Capability
A statistical measure of a process's ability to produce output within preset specifications and quality limits.
Exceed Requirements
To perform beyond the expectations or standards that have been set.
Being In Control
The perception or reality of an individual having the power or management over situations, objects, or people.
Q21: Saturated fats and oils have _ melting
Q31: Henry's Law constant is 0.0013 mol/kg⋅bar and
Q53: Phenanthroline (C<sub>12</sub>H<sub>8</sub>N<sub>2</sub>)can bind to metal ions using
Q60: The chemical formula Ga<sub>2</sub>O<sub>3</sub> indicates<br>A) two atoms
Q67: Which of the following statements is true
Q72: What is the total number of electrons
Q84: Identify from the following list of molecules
Q110: Explain the treatment named boron neutron capture
Q117: How many protons,electrons,and neutrons,respectively,does <sup>58</sup>Fe have?<br>A) 26,26,32<br>B)
Q142: The average mass of a boron atom