Calculate 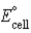 for the electrochemical cell Ag(s) | AgCl(s) | Cl-(aq,1.0 M) || Cu2+(aq,1.0 M) | Cu(s) . The standard reduction potentials are as follows:
for the electrochemical cell Ag(s) | AgCl(s) | Cl-(aq,1.0 M) || Cu2+(aq,1.0 M) | Cu(s) . The standard reduction potentials are as follows:
Cu2+(aq) + 2 e- → Cu(s)
E° = +0.337 V
AgCl(s) + e- → Ag(s) + Cl-(aq)
E° = +0.222 V
Definitions:
Distinguish
To recognize or note the difference between two or more things, often used in the context of chemical testing to identify substances.
Reaction Sequence
A series of chemical reactions where the product of one reaction serves as the reactant for the next.
Phospholipid
A lipid molecule that is a major component of cell membranes, consisting of two fatty acids, a phosphate group, and a glycerol backbone.
Alkaloid
A class of naturally occurring organic compounds that mostly contain basic nitrogen atoms.
Q5: Which of the following statements is true
Q13: Which of the following reactions is a
Q28: Which of the following statements about the
Q35: What is the name of the given
Q35: The products of the reaction of strontium
Q42: Write a balanced chemical equation for the
Q44: The Ostwald process is an industrial method
Q70: What is the equilibrium hydronium ion concentration
Q70: Write the expression for K<sub>p</sub> for the
Q169: How many atoms are represented by one