The value of E°cell is 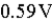 for the following reaction: Cl2(g) + 2 Fe2+(aq) → 2 Fe3+(aq) + 2 Cl-(aq)
for the following reaction: Cl2(g) + 2 Fe2+(aq) → 2 Fe3+(aq) + 2 Cl-(aq)
Calculate the value of E°cell for the reaction below.
Cl-(g) + Fe3+(aq) → Fe2+(aq) + ½ Cl2(g)
Definitions:
Ordered Data Set
A collection of objects or values arranged in a sequence following a specific order, usually numerical or alphabetical.
Observations
Individual data points or measurements collected during a study or experiment.
Histogram
A graphical representation of the distribution of numerical data, showing the frequency of data points within successive intervals.
Proportion
The ratio of a part to the whole, often expressed as a fraction, decimal, or percentage.
Q2: Which of the following solutions has the
Q11: If the complete consumption of 10.3 g
Q11: The K<sub>sp</sub> of BaSO<sub>4</sub> is 1.1 ×
Q14: Which of the following is true of
Q18: For the zero-order reaction A → B
Q19: Identify the product of the hydrogenation of
Q21: Which of the following expressions for K
Q33: If a metal crystallizes in a body-centered
Q39: What is the mass of H<sub>2</sub>SO<sub>4</sub> in
Q45: Using the given data,determine ΔrG° at 500.0