Use the following standard reduction potentials to determine which species is the strongest oxidizing agent. Fe2+(aq) + 2 e- → Fe(s) ; E° = -0.41 V
Pt2+(aq) + 2 e- → Pt(s) ; E° = 1.18 V
Cr2O72-(aq) + 14 H+(aq) + 6 e- → 2 Cr3+(aq) + 7 H2O( 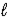 ) ; E° = 1.33 V
) ; E° = 1.33 V
Definitions:
ROI
Return on investment (ROI) measures the gain or loss generated on an investment relative to the amount of money invested.
ROI
An evaluation tool applied to determine the profitability or efficiency of a single investment or to analyze and compare the efficacy of multiple investments.
Margin
The difference between the selling price of a product and its cost, often expressed as a percentage of the selling price.
Division
A distinct part of a larger company or organization that operates semi-independently, focusing on a specific set of products, services, or market segment.
Q12: How many rings are found in a
Q15: Which of the following reactions is responsible
Q16: Which of the following statements is true
Q22: How many unpaired electrons are found in
Q25: Oxides of the alkaline earth family form
Q27: What is the pH of a buffer
Q38: The carbon-carbon bonding of cyclopropane is shown
Q58: Which of the following species have geometric
Q61: For a chemical reaction,the activation energy for
Q162: The number of protons in the nucleus