Calculate 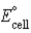 for the electrochemical cell Ag(s) | AgCl(s) | Cl-(aq,1.0 M) || Cu2+(aq,1.0 M) | Cu(s) . The standard reduction potentials are as follows:
for the electrochemical cell Ag(s) | AgCl(s) | Cl-(aq,1.0 M) || Cu2+(aq,1.0 M) | Cu(s) . The standard reduction potentials are as follows:
Cu2+(aq) + 2 e- → Cu(s)
E° = +0.337 V
AgCl(s) + e- → Ag(s) + Cl-(aq)
E° = +0.222 V
Definitions:
Equity Method
An accounting technique used for investments in which the investor has significant influence over the investee but does not exert full control, reflecting the investor's share of the investee's profits and losses.
Cost Method
An accounting method used to value certain investments based on their original purchase cost, without reflecting market value changes until realized.
Economic Interest
A stake or concern in an economic entity or activity, often referring to the level of investment or involvement an individual or organization possesses in a company or project.
Equity Method
An accounting technique used by companies to record their investments in other companies when they have significant influence but not full control.
Q3: Given the equilibrium constants for the following
Q12: Calculate the activation energy,E<sub>a</sub>,for N<sub>2</sub>O<sub>5</sub>(g)→ 2 NO<sub>2</sub>(g)+
Q33: Which of the following statements is true
Q34: Which of the following statements is INCORRECT?<br>A)
Q41: The primary source material for the commercial
Q45: For the reaction N<sub>2</sub>O<sub>4</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="For
Q49: What is the mole fraction of urea,CO(NH<sub>2</sub>)<sub>2</sub>,in
Q50: A 15 meter by 12 meter pool
Q75: Which of the following is true of
Q106: The symbol for the element strontium is<br>A)