For the reaction given below,ΔH0 = −1516 kJ at 25°C and ΔS0 = −432.8 J/K at 25°C.This reaction is spontaneous _____. SiH4(g) + 2 O2(g) → SiO2(s) + 2 H2O 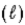
Definitions:
Average Collection Period
The typical duration a business must wait to collect payments from its customers for goods or services sold on a credit basis.
Financial Data
Refers to information related to the financial performance of an entity, including income, expenses, assets, and liabilities.
Sales On Account
Transactions where goods or services are sold and payment is deferred to a future date, essentially selling on credit.
Cost Of Goods Sold
The costs directly connected with generating the goods a firm sells to its customers.
Q2: Aerobic respiration links the oxidation of glucose
Q6: Thermodynamics can be used to determine all
Q24: A metal crystallizes in a face-centered cubic
Q33: If a metal crystallizes in a body-centered
Q41: Calculate the value of the reaction quotient,Q,for
Q53: What is the length of the diagonal
Q64: What is the H<sub>3</sub>O<sup>+</sup> concentration in 0.0072
Q77: Excess Ag<sub>2</sub>SO<sub>4</sub>(s)is placed in water at 25
Q78: The chemical formula of _ is K<sub>2</sub>[PtCl<sub>4</sub>].<br>A)
Q81: Use your knowledge of group properties to