Calculate ΔrG° for the reaction below at 425 °C, 2 HI(g) 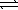 H2(g) + I2(g) ; K = 0.018.(R = 8.314 J/K⋅mol)
H2(g) + I2(g) ; K = 0.018.(R = 8.314 J/K⋅mol)
Definitions:
No-Par Common Stock
No-Par common stock is stock issued without a par value, meaning its value is determined by the price investors are willing to pay in the market, rather than a set face value.
Stated Value
Stated value refers to a fixed per-share value assigned by a corporation to its no-par value stock, often used in accounting and financial reporting.
Participating Right
A feature of certain securities that allows the holder to receive dividends or other benefits in addition to the basic rights associated with the security.
Outstanding Stock
Shares of a corporation that have been issued and are currently held by investors, including public shareholders and company insiders.
Q16: If one of the factors determining the
Q40: What concentration of silver nitrate (in ppm)is
Q41: The Henry's law constant for O<sub>2</sub> in
Q44: A 25.00-mL sample of propionic acid,HC<sub>3</sub>H<sub>5</sub>O<sub>2</sub>,of unknown
Q49: Which of the following cations would be
Q50: Which are the Brønsted-Lowry bases in the
Q53: What is the length of the diagonal
Q56: A 12.0% sucrose solution by mass has
Q77: All of the following complexes will be
Q81: Use your knowledge of group properties to