Given the following reactions, AgBr(s) 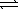 Ag+(aq) + Br-(aq)
Ag+(aq) + Br-(aq)
Ksp = 5.4 × 10-13
Ag+(aq) + 2 CN-(aq) 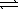 Ag(CN) 2-(aq)
Ag(CN) 2-(aq)
Kf = 1.2 × 1021
Determine the equilibrium constant for the reaction below.
AgBr(s) + 2 CN-(aq) 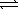 Ag(CN) 2-(aq) + Br-(aq)
Ag(CN) 2-(aq) + Br-(aq)
Definitions:
Night Owls
Individuals who prefer staying up late and are more alert and energetic in the evening and at night compared to the morning.
Lark
A morning person, often energetic and active at the start of the day.
Evolutionary Perspective
A theoretical approach in psychology that explains human thoughts, feelings, and behaviors through the lens of natural selection and adaptation.
Universal Trait
Characteristics of personality that are considered to exist within every individual across different cultures and societies.
Q4: Which of the following is the monomer
Q5: What is the pH of a solution
Q22: The symbol Q is called the _.
Q23: Radioactive isotopes decay by _-order kinetics.
Q25: How many structural isomers possessing the alcohol
Q37: The metal vanadium crystallizes in a body-centered
Q61: For a chemical reaction,the activation energy for
Q62: Which of the following sets of elements
Q64: At a temperature (in kelvin units)of _,the
Q75: Use the following standard reduction potentials to