Given the two equilibria below, Ag(NH3) 2+(aq) 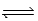 Ag+(aq) + 2NH3(aq) ; Kd = 5.9 × 10-8
Ag+(aq) + 2NH3(aq) ; Kd = 5.9 × 10-8
AgCN(s) 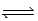 Ag+(aq) + CN−(aq) ; Ksp =
Ag+(aq) + CN−(aq) ; Ksp = 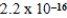 what is K for the following equilibrium?
what is K for the following equilibrium?
AgCN(s) + 2NH3(aq)  Ag(NH3) 2+(aq) + CN-(aq)
Ag(NH3) 2+(aq) + CN-(aq)
Definitions:
Stock Listed
A company's shares that are available for trading on a public stock exchange.
Share Repurchase
A corporate action in which a company buys back its own shares from the marketplace, often to increase the value of remaining shares.
Cash Dividend Payment
A cash dividend payment is a distribution of profits by a corporation to its shareholders in the form of cash.
Outstanding Stock
The total shares of stock that are owned by investors, including shares held by institutional investors and company insiders.
Q6: Which equation represents the number of atoms
Q12: What is the molar solubility of solid
Q12: Arrange the following in order of increasing
Q13: The reaction kinetics for a certain reaction
Q31: Sulfur dioxide has a vapor pressure of
Q42: Which element has the following ground state
Q46: At 80.0 °C,water has an equilibrium vapor
Q51: You have a 30.0 L cylinder of
Q56: What mass of MgO would be produced
Q62: Aqueous solutions of ammonia (NH<sub>3</sub>)and hydrogen cyanide