Given the following equilibrium constants, Zn4IO3) 2 Ksp = 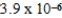 Zn(NH3) 42+ Kf =
Zn(NH3) 42+ Kf = 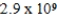 determine K for the dissolution of the sparingly soluble salt Zn(IO3) 2 in aqueous ammonia (shown below) .
determine K for the dissolution of the sparingly soluble salt Zn(IO3) 2 in aqueous ammonia (shown below) .
Zn(IO3) 2(s) + 4NH3(aq) ⇌ Zn(NH3) 42+(aq) + 2IO3-(aq)
Definitions:
Sherman Act
A landmark federal statute in U.S. antitrust law passed in 1890 which prohibits monopolistic practices and has been used to regulate competition among enterprises.
Reliable Guidance
Advice or instructions that are dependable and trustworthy, often based on expertise or authoritative sources.
Chicago School Theorists
Chicago School Theorists are economists and legal scholars associated with the University of Chicago, known for their advocacy of free-market principles and minimal government intervention.
Antitrust Policy
comprises laws and regulations designed to promote competition and prevent monopolies, ensuring fair business practices.
Q1: For the reaction A + 2B →
Q4: What is the expression for K<sub>c</sub> for
Q11: Assuming ideal behavior,which of the following aqueous
Q12: Calculate the activation energy,E<sub>a</sub>,for N<sub>2</sub>O<sub>5</sub>(g)→ 2 NO<sub>2</sub>(g)+
Q21: Biodiesel is a mixture of:<br>A) fats and
Q36: What is the hydroxide-ion concentration in a
Q43: Which of the following statements is true
Q44: A salt with a 1:1 ratio of
Q48: When a secondary alcohol is oxidized,the product
Q70: What is the equilibrium hydronium ion concentration