Given the following reactions, AgBr(s) 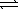 Ag+(aq) + Br-(aq)
Ag+(aq) + Br-(aq)
Ksp = 5.4 × 10-13
Ag+(aq) + 2 CN-(aq) 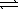 Ag(CN) 2-(aq)
Ag(CN) 2-(aq)
Kf = 1.2 × 1021
Determine the equilibrium constant for the reaction below.
AgBr(s) + 2 CN-(aq) 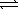 Ag(CN) 2-(aq) + Br-(aq)
Ag(CN) 2-(aq) + Br-(aq)
Definitions:
ERP
Enterprise Resource Planning, a type of software that organizations use to manage and integrate important parts of their businesses, including finance, supply chain, operations, and human resources.
Net Requirements
The total quantity needed of a particular item, considering current inventory levels and future orders but excluding any on-hand inventory.
Gross Requirements
represent the total amount of materials or products required to meet production demands, before accounting for any existing inventory.
Product A
A placeholder name for a specific product, typically used in examples or theoretical discussions.
Q2: Which of the following substances is never
Q6: Which equation depicts the hydrogen phosphate ion
Q6: The cell potential of the following electrochemical
Q7: What is the molar solubility of Mn(OH)<sub>2</sub>(s)in
Q15: The bandgap of ZnTe is 218 kJ/mol.What
Q28: Which of the following statements about the
Q30: Silver chloride adopts the sodium chloride (rock
Q50: The Arrhenius equation, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="The Arrhenius
Q68: Which of the following is the general
Q79: Colloids represent a state intermediate between a