Given the following equilibrium constants, Zn4IO3) 2 Ksp = 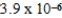 Zn(NH3) 42+ Kf =
Zn(NH3) 42+ Kf = 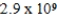 determine K for the dissolution of the sparingly soluble salt Zn(IO3) 2 in aqueous ammonia (shown below) .
determine K for the dissolution of the sparingly soluble salt Zn(IO3) 2 in aqueous ammonia (shown below) .
Zn(IO3) 2(s) + 4NH3(aq) ⇌ Zn(NH3) 42+(aq) + 2IO3-(aq)
Definitions:
Movie Tickets
Certificates or electronic codes that grant the holder the right to admission for viewing a film at a cinema.
Complementarity
A relationship between two goods where the use of one increases the value or demand for the other.
Equilibrium Price
The market price at which the quantity of goods supplied is equal to the quantity of goods demanded, also known as the market-clearing price.
Supply and Demand Functions
Mathematical expressions that describe the relationship between the supply of and demand for goods or services within a market.
Q4: In general,as temperature increases,the rate of a
Q4: Which of the following is the common
Q16: The metal sodium crystallizes in a body-centered
Q30: If 0.357 g of CH<sub>4</sub> gas is
Q34: Which of the following statements is INCORRECT?<br>A)
Q48: Calculate the standard reduction potential for the
Q58: If the ratio of acid to base
Q58: What is the name given to a
Q71: Calculate the pH of a 1.7 M
Q74: If 20 mL of 0.10 M NaOH