Given the equilibrium constants for the equilibria, H2O(l) 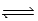 H3O+(aq) ; K =
H3O+(aq) ; K = 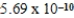 2CH3COOH(aq) + 2H2O(l)
2CH3COOH(aq) + 2H2O(l) 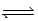 2CH3COO−(aq) + 2H3O+(aq) ; K =
2CH3COO−(aq) + 2H3O+(aq) ; K = 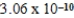 determine Kc for the following equilibrium.
determine Kc for the following equilibrium.
CH3COOH(aq) + NH3(aq) 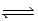 CH3COO−(aq) + NH4+(aq)
CH3COO−(aq) + NH4+(aq)
Definitions:
Bipolar Disorder
Bipolar disorder is a mental health condition marked by extreme mood swings, including emotional highs (mania or hypomania) and lows (depression).
Major Depressive Disorder
A clinical condition characterized by persistent sadness, lack of interest in activities, and other symptoms that interfere with daily functioning.
Serotonin And Norepinephrine
Neurotransmitters involved in regulating mood, anxiety, happiness, and alertness, often targeted by certain medications to treat depression and other mood disorders.
Paxil
A medication known generically as paroxetine, used primarily to treat depression, anxiety disorders, and related conditions.
Q13: Cesium crystallizes in the body-centered cubic system.If
Q18: For which of the following reactions will
Q20: Write a balanced net ionic equation for
Q22: Carbonic acid is a diprotic weak acid.Which
Q23: According to the National Institute of Standards
Q24: What is the freezing point of an
Q25: The volume of a 32.4% (by mass)solution
Q27: Which of the following statements is INCORRECT?<br>A)
Q42: Calculate the mass of coal that produces
Q79: What volume of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="What volume