What is the equilibrium constant for the following reaction, HCO2H(aq) + CN-(aq) 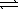 HCO2-(aq) + HCN(aq)
HCO2-(aq) + HCN(aq)
And does the reaction favor the formation of reactants or products? The acid dissociation constant,Ka,for HCO2H is 1.8 × 10-4 and the acid dissociation constant for HCN is 4.0 × 10-10.
Definitions:
Post-closing Trial Balance
A list of all accounts and their balances after closing entries are made, used to verify the equality of debits and credits.
Balance Sheet Accounts
Accounts that are reported on the balance sheet and include assets, liabilities, and equity sections, reflecting the financial position of a company at a specific time.
Owner's Equity
The residual interest in the assets of a business after deducting its liabilities.
Total Liabilities
The sum of all financial obligations a company owes to outside parties, including loans, accounts payable, and mortgages.
Q9: Which of the following is not a
Q14: Which of the following reactions does not
Q18: For a particular liquid,raising its temperature from
Q35: The products of the reaction of strontium
Q40: The change in entropy for any process
Q52: Which of the following relationships is not
Q58: Aluminum is resistant to corrosion because:<br>A) its
Q63: When a glass tube with a small
Q68: When a water molecule forms a hydrogen
Q69: Consider the reaction below. 2 HF(g) <img