Multiple Choice
Given the equilibrium constants for the equilibria, H2O(l) 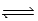 H3O+(aq) ; K =
H3O+(aq) ; K = 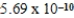 2CH3COOH(aq) + 2H2O(l)
2CH3COOH(aq) + 2H2O(l) 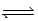 2CH3COO−(aq) + 2H3O+(aq) ; K =
2CH3COO−(aq) + 2H3O+(aq) ; K = 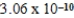 determine Kc for the following equilibrium.
determine Kc for the following equilibrium.
CH3COOH(aq) + NH3(aq) 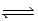 CH3COO−(aq) + NH4+(aq)
CH3COO−(aq) + NH4+(aq)
Understand different behaviors within groups and teams, including competitive and cooperative behaviors.
Identify strategies to counteract negative group behaviors such as social loafing.
Comprehend the role of task and maintenance functions in effective work group performance.
Understand the significance of team diversity and individual contributions to team efficacy.
Definitions:
Related Questions
Q3: Which of the following expressions does not
Q9: What is the sign of ΔH (system)and
Q17: Which of the following are the expected
Q22: The reaction,A + 2B → B<sub>2</sub> +
Q28: What volume of O<sub>2</sub>,measured at 24.0°C and
Q49: A student analyzed a first-order reaction and
Q53: When a Lewis acid combines with a
Q59: The pH of a solution at 25
Q72: What is the total number of electrons
Q80: Which of the following calcium compounds is