When gaseous carbon monoxide and hydrogen are combined in a sealed vessel and heated they will eventually form an equilibrium mixture of reactants and products according to the balanced chemical equilibrium below. CO(g) + 3H2(g) 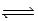 CH4(g) + H2O(g)
CH4(g) + H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve B? 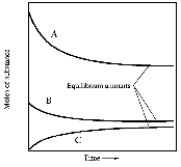
Definitions:
Unlock Potential
The process by which individuals or groups are encouraged and enabled to achieve greater capabilities or achieve new levels of performance.
Human Resource Management
The strategic approach to the effective management of people in an organization, in a way that can help their business gain a competitive advantage.
Organisational Mission
A statement that defines the purpose, goals, and values of an organization, guiding its strategic direction and decisions.
Energetic Workforce
A group of employees who demonstrate vitality, enthusiasm, and a high level of activity in their work.
Q12: Point D on the phase diagram is
Q16: Calculate the pH of the following aqueous
Q21: Ethanol has an enthalpy of vaporization of
Q25: If a cube of ice at 0
Q35: Which of the following complex ions has
Q41: The primary source material for the commercial
Q52: Write balanced reduction and oxidation half-reactions for
Q62: What is the outer shell ground state
Q63: What mass of sodium hydroxide must be
Q78: Which of the following is true for