Consider the following equilibrium: C2H6(g) + C5H12(g) 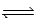 CH4(g) + C6H14(g) ; Kp = 9.57 at 500 K
CH4(g) + C6H14(g) ; Kp = 9.57 at 500 K
Suppose 22.7 g each of CH4,C2H6,C5H12,and C6H14 are placed in a 35.0-L reaction vessel at 500 K.Which of the following statements is correct?
Definitions:
Direct Labor-Hours
The total hours worked by employees directly involved in the production process, used as a measure for allocating labor costs to products or services.
Variable Overhead
Costs of indirect manufacturing that change with the level of production output, such as utilities for the manufacturing plant.
Efficiency Variance
The difference between the actual amount of input (time, materials, etc.) used in production and the standard amount expected to be used.
Direct Labor-Hours
The sum of the hours spent by workers directly manufacturing a product.
Q12: According to the cell notation below,which of
Q30: Which of the following substances will have
Q44: To form a molecule with a tetrahedral
Q53: What mass of Zn(NO<sub>3</sub>)<sub>2</sub> must be diluted
Q54: Consider the K<sub>a</sub> values for the following
Q68: What is the maximum hydroxide-ion concentration that
Q74: If two or more species have the
Q81: Hydrofluoric acid has a pK<sub>a</sub> value of
Q86: Which of the following pairs of compounds
Q87: What is the conjugate base of [Fe(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>(aq)?<br>A)