Nitrogen trifluoride decomposes at to form nitrogen and fluorine gases according to the following equation: 2NF3(g) 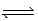 N2(g) + 3F2(g)
N2(g) + 3F2(g)
2) 50-L reaction vessel is initially charged with 1.22 mol of NF3 and allowed to come to equilibrium at 800 K.Once equilibrium is established,the reaction vessel is found to contain 0.0194 mol of N2.What is the value of Kp at this temperature? (R = 0.0821 L⋅atm/mol⋅K)
Definitions:
Financial Rewards
Monetary benefits given to employees or individuals as recognition for their work performance, achievements, or contributions.
Meaningfulness
The significance or value that work holds for an individual, often contributing to motivation and job satisfaction.
Heading
The title or caption of a section within a document, used to introduce or summarize its content.
Material
Physical substances from which things can be made or actual constituents that have significant importance or relevance to a subject or issue.
Q2: Which of the following units are consistent
Q9: Which of the following compounds is expected
Q13: An unknown gaseous hydrocarbon contains 85.63% C.Its
Q14: What is the freezing point of a
Q32: When the given oxidation-reduction reaction in an
Q45: Which of the following reactions describes the
Q52: What is the total volume of gases
Q62: For the following gas-aqueous liquid equilibrium for
Q70: Which of the following steps must occur
Q70: A 22.4 L high pressure reaction vessel