At 25 °C,0.138 mg AgBr dissolves in 10.0 L of water.What is the equilibrium constant for the reaction below? AgBr(s) 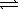 Ag+(aq) + Br-(aq)
Ag+(aq) + Br-(aq)
Definitions:
Mental Representation
An internal image or symbol of external reality, created by the brain.
Modes of Thought
Refers to the different ways in which individuals think and perceive the world, influenced by their cognitive abilities, experiences, and cultural backgrounds.
Synaptogenesis
The formation of synapses between neurons in the brain, a crucial process for learning, memory, and neural development.
Q2: Which of the following mathematical expressions is
Q9: Which of the following compounds was used
Q15: Based on the following data,what is the
Q19: What is the minimum concentration of Cd<sup>2+</sup>
Q37: Silicon is purified in a process called
Q45: What process occurs when the temperature of
Q50: Calculate the charge,in coulombs,is required to deposit
Q51: You have a 30.0 L cylinder of
Q62: A flexible vessel contains 49.00 L of
Q70: Which of the following steps must occur