Multiple Choice
At a given temperature,0.0664 mol N2O4(g) is placed in a 1.00 L flask.After reaching equilibrium,the concentration of NO2(g) is 6.1 × 10-3 M.What is Kc for the reaction below? N2O4(g) 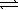 2 NO2(g)
2 NO2(g)
Definitions:
Related Questions
Q5: Aqueous colloidal solutions can be classified as
Q20: A balloon has a volume of 2.52
Q32: Which of the following is the name
Q43: Calculate E°<sub>cell</sub> for the cell for the
Q43: What is the osmotic pressure of an
Q49: The hybridization of the nitrogen atom in
Q58: What is the name given to a
Q73: At 25 °C,all of the following ions
Q74: Write a balanced net ionic equation for
Q79: Colloids represent a state intermediate between a