At 700 K,Kp for the following equilibrium is 5.6 × 10-3. 2HgO(s) 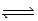 2Hg(l) + O2(g)
2Hg(l) + O2(g)
Suppose 43.1 g of mercury(II) oxide is placed in a sealed 4.00-L vessel at 700 K.What is the partial pressure of oxygen gas at equilibrium? (R = 0.0821 L · atm/(K · mol) )
Definitions:
Lend-Lease Act
A program initiated by the United States during World War II that allowed the U.S. to supply Allied nations with vast amounts of military materiel while avoiding direct involvement in combat.
Military Supplies
Equipment, tools, vehicles, and logistical support materials utilized by armed forces for the purposes of national defense and warfare operations.
Impoverished England
Refers to periods in English history marked by widespread poverty and economic hardship.
Neutrality Acts
Legislation passed in the United States during the 1930s aimed at preventing the nation from being drawn into foreign conflicts by limiting American involvement with countries at war.
Q3: Which of the following chemical equations shows
Q10: Arrange the three common cubic unit cells
Q18: For which of the following reactions will
Q20: At a given temperature,0.0664 mol N<sub>2</sub>O<sub>4</sub>(g)is placed
Q22: The symbol Q is called the _.
Q29: Which of the following is the correct
Q40: What is the hybridization of the central
Q55: For which of the following reactions is
Q57: What is the mole fraction of calcium
Q65: Which of the following bonds can potentially