The equilibrium constant at 25 °C for the dissolution of silver iodide is 8.5 × 10-17. AgI(s) 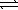 Ag+(aq) + I-(aq)
Ag+(aq) + I-(aq)
If an excess quantity of AgI(s) is added to water and allowed to equilibrate,what is the equilibrium concentration of I-?
Definitions:
Digital Marketing
The promotion of products or brands via one or more forms of electronic media, such as social media, email, and web advertising.
Traditional Marketing
A method of promoting products and services through direct advertising using tools like print ads, television, radio, and direct mail.
Q8: Which of the following is the first
Q12: A molecule will always be polar if
Q19: Write a balanced chemical equation which corresponds
Q19: The dissolution of ammonium nitrate occurs spontaneously
Q45: A solution has a hydroxide ion concentration
Q48: What is the pH of the buffer
Q57: _ is a measure of the degree
Q59: The following equation is known as _
Q64: For a certain overall second-order reaction with
Q70: Which of the following steps must occur