The equilibrium constant (Kc) for the decomposition of ammonium hydrogen sulfide,NH4HS(s) 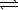 NH3(g) + H2S(g) ,is 1.8 × 10-4 at 25 °C.If excess NH4HS(s) is allowed to equilibrate at 25 °C,what is the equilibrium concentration of NH3?
NH3(g) + H2S(g) ,is 1.8 × 10-4 at 25 °C.If excess NH4HS(s) is allowed to equilibrate at 25 °C,what is the equilibrium concentration of NH3?
Definitions:
Intelligence Scores
Numerical estimations of an individual's intelligence level, often derived from standardized tests.
Biased Tests
Assessments that have systemic errors favoring certain groups over others based on factors unrelated to the skills or knowledge being measured.
Gender Gap
The disparities between males and females in various aspects of society, including economic participation, wages, and education.
Math Grades
Numerical or letter assessments representing a student’s proficiency and understanding in mathematics over a given period.
Q6: The cell potential of the following electrochemical
Q17: If an ionic compound with the formula
Q20: For the first-order decomposition of N<sub>2</sub>O<sub>5</sub> at
Q22: The symbol Q is called the _.
Q30: If 0.357 g of CH<sub>4</sub> gas is
Q37: What mass of solid NaCH<sub>3</sub>CO<sub>2</sub> (molar mass
Q65: A 3.0 g sample of a small
Q65: A 50.00-mL solution of 0.0426 M trimethylamine
Q71: The active ingredients in "strike anywhere" matches
Q73: At 25 °C,all of the following ions