Given the equilibrium constants for the equilibria, NH4+(aq) + H2O(l) 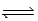 NH3(aq) + H3O+(aq) ; Kc =
NH3(aq) + H3O+(aq) ; Kc = 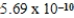 2H2O(l)
2H2O(l) 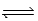 2H3O+(aq) ; Kc =
2H3O+(aq) ; Kc = 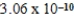 determine Kc for the following equilibrium.
determine Kc for the following equilibrium.
CH3COOH(aq) + NH3(aq) 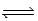 CH3COO−(aq) + NH4+(aq)
CH3COO−(aq) + NH4+(aq)
Definitions:
Passive Voice
A grammatical construction where the subject of the sentence is acted upon by the verb.
"You" Attitude
A communication style that focuses on the needs, interests, or feelings of the audience, often used to create a more engaging or persuasive message.
Situations
Specific circumstances or sets of conditions at a particular time or place.
Conversational Tone
A style of communication that is informal, natural, and similar to everyday spoken language, often used to make written content more relatable.
Q3: What is the molality of a 19.4
Q4: A concentrated hydrochloric acid solution is 37.2%
Q20: For the first-order decomposition of N<sub>2</sub>O<sub>5</sub> at
Q31: If 7 orbitals on one atom overlap
Q41: Which of the following equilibria would not
Q50: Refer to Diagram 9-1.According to molecular orbital
Q62: Nickel crystallizes in a face-centered cubic lattice.The
Q62: What molar ratio of acetic acid to
Q63: What mass of sodium hydroxide must be
Q66: How many lone pairs are present around