Consider the following equilibria. PbBr2(s) 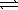 Pb2+(aq) + 2 Br-(aq)
Pb2+(aq) + 2 Br-(aq)
K1 = 6.6 × 10-6
Pb(OH) 2(s) 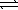 Pb2+(aq) + 2 OH-(aq)
Pb2+(aq) + 2 OH-(aq)
K2 = 1.4 × 10-15
Determine the equilibrium constant,Kc,for the reaction below.
PbBr2(s) + 2 OH-(aq) 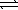 Pb(OH) 2(s) + 2 Br-(aq)
Pb(OH) 2(s) + 2 Br-(aq)
Definitions:
Daily Cash Flow
A financial report showing the net amount of cash that is received or dispersed by a business within a given day.
Receipts
Documents that acknowledge the receipt of goods or the receipt of a transaction.
Fraudulent E-Mails
Deceptive emails sent with the intention of tricking recipients into parting with their money, personal details, or both.
Wire Fraud
A fraudulent scheme to intentionally deprive another of property or honest services via telecommunications or the internet.
Q6: If the ratio of acid to base
Q8: What is the pH of a solution
Q19: The rate constant for a particular reaction
Q24: A metal crystallizes in a face-centered cubic
Q25: The equilibrium constant,K,is always the same within
Q29: The standard entropy for the formation of
Q61: A solution is prepared by dissolving 5.88
Q69: Molecules or ions that can alternately behave
Q73: If 25 mL of 0.750 M HCl
Q86: What is the equilibrium pH of an