The standard enthalpy of formation of ammonia is -46.1 kJ/mol.
1/2 N2(g)+ 3/2 H2(g) 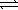 NH3(g)
NH3(g)
Commercially,the reaction is carried out at high temperatures.Using your knowledge of kinetics and equilibrium,explain an advantage and a disadvantage of synthesizing ammonia at high temperatures.
Definitions:
Ketonuria
The presence of ketone bodies in the urine, often a sign of insufficient insulin in individuals with diabetes.
Diabetes
A metabolic disease characterized by high blood sugar levels over a prolonged period, resulting from defects in insulin production, insulin action, or both.
Daily Injections
The routine of administering drugs or other substances into the body via a needle and syringe on a daily basis.
Juvenile Hypothyroidism
A condition in which the thyroid gland of children produces insufficient thyroid hormone, affecting growth and development.
Q1: What is the hybridization of the central
Q1: For the reaction A + 2B →
Q2: Which of the following mathematical expressions is
Q2: Which of the following solutions has the
Q8: Consider the titration of 300.0 mL of
Q13: An unknown gaseous hydrocarbon contains 85.63% C.Its
Q30: Bauxite ore is the primary source material
Q55: How many milliliters of 11.7 M H<sub>2</sub>SO<sub>4</sub>
Q67: List all the intermolecular forces present in
Q75: What is the minimum mass of Na<sub>2</sub>CO<sub>3</sub>