When gaseous carbon monoxide and hydrogen are combined in a sealed vessel and heated they will eventually form an equilibrium mixture of reactants and products according to the balanced chemical equilibrium below. CO(g) + 3H2(g) 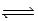 CH4(g) + H2O(g)
CH4(g) + H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve B? 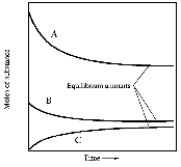
Definitions:
Medicare
A federal health insurance program in the United States for people aged 65 and older, as well as for some younger people with disabilities.
Earnings Percent
Earnings percent refers to the proportion or percentage of income earned from an investment, work, or business operation relative to the initial cost or investment size.
Workforce
The total number of people employed or available for employment or work in a country, organization, or specific industry.
Crash Of 1929
The severe plunge in stock market prices that initiated the Great Depression, beginning in late October 1929.
Q2: For which of the following molecules and
Q4: Mount Everest rises to a height of
Q6: As pure molecular solids,which of the following
Q12: According to the cell notation below,which of
Q24: An aqueous solution with a pH of
Q31: If 7 orbitals on one atom overlap
Q39: What is the hybridization of the nitrogen
Q44: A 25.00-mL sample of propionic acid,HC<sub>3</sub>H<sub>5</sub>O<sub>2</sub>,of unknown
Q57: H<sub>3</sub>PO<sub>3</sub> is a diprotic weak acid.What is
Q61: Rubidium iodide (molar mass 212.4 g/mol)has a