Which of the following is always true for a reaction where Kc is 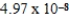 at 25°C?
at 25°C?
Definitions:
Outstanding Call Options
Call options that have been sold but not yet exercised, representing potential claims on the underlying security.
Efficient-market Hypothesis
The theory that all known information is already reflected in stock prices, making it impossible to consistently achieve higher returns than the overall market.
Security Prices
The current cost or value at which a particular security, such as stocks, bonds, or options, can be bought or sold in the market.
Empirical Validity
The extent to which a theoretical concept is supported by real-world evidence or data.
Q7: What is the equilibrium partial pressure of
Q12: Which of the following statements concerning octahedral
Q16: What is the total number of valence
Q24: What is the freezing point of an
Q26: A catalyst _.<br>A) is used up in
Q35: A solution consisting of 0.278 mol of
Q38: Hyperventilation can cause your blood pH to
Q63: Refer to Diagram 9-1.According to molecular orbital
Q70: A 22.4 L high pressure reaction vessel
Q78: Which list contains the three most abundant