Nitrogen trifluoride decomposes at to form nitrogen and fluorine gases according to the following equation: 2NF3(g) 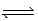 N2(g) + 3F2(g)
N2(g) + 3F2(g)
2) 50-L reaction vessel is initially charged with 1.22 mol of NF3 and allowed to come to equilibrium at 800 K.Once equilibrium is established,the reaction vessel is found to contain 0.0194 mol of N2.What is the value of Kp at this temperature? (R = 0.0821 L⋅atm/mol⋅K)
Definitions:
Energy Fields
In some holistic and alternative medicine practices, invisible fields believed to surround and penetrate the human body, influencing health and healing.
Hydromorphone
A potent opioid analgesic medication used to relieve moderate to severe pain.
Naloxone (Narcan)
A medication used to counter the effects of opioid overdose, rapidly reversing the life-threatening respiratory depression.
Music Therapy
A therapeutic approach that uses music interventions to accomplish individualized goals within a therapeutic relationship.
Q11: If the complete consumption of 10.3 g
Q17: Which of the following is a colligative
Q26: A catalyst _.<br>A) is used up in
Q43: An impure sample of sodium carbonate,Na<sub>2</sub>CO<sub>3</sub>,is titrated
Q56: The pH of aqueous 0.10 M pyridine
Q66: Which of the following statements is true
Q73: For the reaction 2NO(g)+ O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg"
Q75: Which of the following is true of
Q77: When the following oxidation-reduction reaction in acidic
Q81: A pressure of 1.00 atm has a