A 3.50-mol sample of HI is placed in a 1.00-L vessel at 460°C,and the reaction system is allowed to come to equilibrium.The HI partially decomposes,forming 0.266 mol H2 and 0.266 mol I2 at equilibrium.What is the equilibrium constant Kc for the following reaction at 460°C? ½ H2(g) + ½ I2(g) 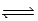 HI(g)
HI(g)
Definitions:
Pure Competition
A market structure characterized by a large number of small firms, homogeneous products, and easy entry and exit, leading to price taking behavior.
Long Run
A period in which all inputs can be adjusted by firms, allowing new firms to enter or exit the industry.
Constant-cost Industry
An industry where the input prices and costs of production do not change as the industry output changes.
Supply Curve
A graphical representation showing the relationship between the price of a good and the quantity supplied at those prices.
Q2: Which of the following solutions has the
Q21: Which of the compounds below is not
Q21: Ethanol has an enthalpy of vaporization of
Q22: Carbonic acid is a diprotic weak acid.Which
Q24: The rate constant of a first-order decomposition
Q49: What is the pH of a solution
Q49: What is the mole fraction of urea,CO(NH<sub>2</sub>)<sub>2</sub>,in
Q56: Refer to Diagram 9-1.Consider the molecules B<sub>2</sub>,C<sub>2</sub>,N<sub>2</sub>
Q56: A 12.0% sucrose solution by mass has
Q73: Which of the following statements is INCORRECT?<br>A)