Multiple Choice
The equilibrium constant at 25 °C for the dissolution of silver iodide is 8.5 × 10-17. AgI(s) 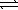 Ag+(aq) + I-(aq)
Ag+(aq) + I-(aq)
If an excess quantity of AgI(s) is added to water and allowed to equilibrate,what is the equilibrium concentration of I-?
Definitions:
Related Questions
Q2: For which of the following molecules and
Q10: As pure molecular solids,which of the following
Q13: The change in energy accompanying the equation
Q15: The molar mass of an unknown gas
Q21: Write a balanced half-reaction for the reduction
Q38: Quartz is a pure crystalline form of
Q41: Which of the following compounds has a
Q55: In which of the following solutions would
Q60: Consider the reaction H<sub>2</sub> + I<sub>2</sub> <img
Q65: A 3.0 g sample of a small