The equilibrium constant (Kc) for the decomposition of ammonium hydrogen sulfide,NH4HS(s) 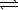 NH3(g) + H2S(g) ,is 1.8 × 10-4 at 25 °C.If excess NH4HS(s) is allowed to equilibrate at 25 °C,what is the equilibrium concentration of NH3?
NH3(g) + H2S(g) ,is 1.8 × 10-4 at 25 °C.If excess NH4HS(s) is allowed to equilibrate at 25 °C,what is the equilibrium concentration of NH3?
Definitions:
Labor-Hour
A unit of measure representing one hour of work by an employee, often used in costing and budgeting processes.
Variable Overhead Efficiency Variance
The difference between the actual variable overhead incurred and the standard variable overhead allocated, based on the actual input of the allocation base.
Fixed Component
A cost that does not change with the increase or decrease in the amount of goods or services produced or sold.
Predetermined Overhead Rate
An established rate used to apply manufacturing overhead costs to products, calculated in advance based on expected costs and activity levels.
Q5: On a relative basis,the weaker the intermolecular
Q10: Arrange the three common cubic unit cells
Q21: Which of the following expressions for K
Q22: The reaction,A + 2B → B<sub>2</sub> +
Q28: Which of the following statements about the
Q50: Nickel has a face-centered cubic cell,and its
Q54: The following equation represents the partial combustion
Q62: Nickel crystallizes in a face-centered cubic lattice.The
Q71: Calculate the pH of a 1.7 M
Q75: Use the following standard reduction potentials to