Multiple Choice
Given the equilibrium constants for the equilibria, NH4+(aq) + H2O(l) 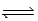 NH3(aq) + H3O+(aq) ; Kc =
NH3(aq) + H3O+(aq) ; Kc = 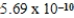 2H2O(l)
2H2O(l) 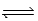 2H3O+(aq) ; Kc =
2H3O+(aq) ; Kc = 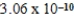 determine Kc for the following equilibrium.
determine Kc for the following equilibrium.
CH3COOH(aq) + NH3(aq) 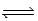 CH3COO−(aq) + NH4+(aq)
CH3COO−(aq) + NH4+(aq)
Definitions:
Related Questions
Q4: The temperature of a specific amount of
Q11: If the complete consumption of 10.3 g
Q13: Which of the following elements is able
Q19: Which one of the following statements is
Q25: The equilibrium constant,K,is always the same within
Q47: Which of the following expressions correctly describes
Q54: Gold and platinum are commonly used as
Q55: How many milliliters of 11.7 M H<sub>2</sub>SO<sub>4</sub>
Q64: Suppose 50.00 mL of 2.0 × 10<sup>-5</sup>
Q70: What is the vapor pressure at 20°C