Consider the following equilibria. PbBr2(s) 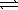 Pb2+(aq) + 2 Br-(aq)
Pb2+(aq) + 2 Br-(aq)
K1 = 6.6 × 10-6
Pb(OH) 2(s) 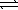 Pb2+(aq) + 2 OH-(aq)
Pb2+(aq) + 2 OH-(aq)
K2 = 1.4 × 10-15
Determine the equilibrium constant,Kc,for the reaction below.
PbBr2(s) + 2 OH-(aq) 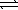 Pb(OH) 2(s) + 2 Br-(aq)
Pb(OH) 2(s) + 2 Br-(aq)
Definitions:
Unexpected Returns
Returns on an investment that exceed what is predicted by models or expected based on historical trends, often caused by unforeseen factors or events.
Expected Returns
The anticipated amount of profit or loss an investor predicts to receive from an investment, taking into account the possibility of fluctuating values.
Unsystematic Risk
The hazard pertaining to an individual business or field, which can be reduced by diversifying assets.
Systematic Risk
A risk pervasive throughout the whole market or a specific segment of it, not reducible by investment diversification.
Q5: For a certain reaction of the general
Q27: Which of the following statements is INCORRECT?<br>A)
Q29: Which one of the following elements is
Q37: A statement of Avogadro's hypothesis is that
Q41: The Henry's law constant for O<sub>2</sub> in
Q55: For which of the following reactions is
Q58: Aluminum is resistant to corrosion because:<br>A) its
Q61: For a chemical reaction,the activation energy for
Q62: For the following gas-aqueous liquid equilibrium for
Q73: The solubility of 1-pentanol in water is