What is the overall order of the reaction NO(g) + O3(g) → NO2(g) + O2(g) if the reaction proceeds via the rate expression given below. 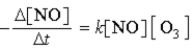
Definitions:
Michigan Football
The intercollegiate football program at the University of Michigan, known for its rich history and contributions to the sport of American football.
Gain Yards
In sports, particularly in American football, it refers to the advancement of the ball down the field to gain distance towards the opponent's end zone.
Probability .70
A statistical measure indicating a 70% chance of a specified event occurring.
Nash Equilibrium
A situation in a non-cooperative game where each player's strategy is optimal given the strategies of all other players.
Q2: Which of the following units are consistent
Q9: A first-order chemical reaction is observed to
Q15: What is the formal charge of the
Q25: The concentration of calcium carbonate in a
Q32: The standard free energy change for a
Q37: A 100 mL sample of water is
Q38: The pressure of O<sub>2</sub> in a 15.0
Q41: Which of the following equilibria would not
Q42: Calculate the mass of coal that produces
Q75: Which of the following is a correct