Multiple Choice
What is a possible set of quantum numbers for an unpaired electron in the orbital box diagram below? 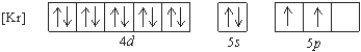
Understand the varying formats of résumés and the purposes of each.
Recognize how to minimize the impacts of frequent job changes and gaps in employment on a résumé.
Identify the components that emphasize growth and achievements within a consistent employment history.
Describe common résumé mistakes and their repercussions on job application outcomes.
Definitions:
Related Questions
Q15: Elementary steps in a reaction mechanism often
Q19: Which type of experiment demonstrates that light
Q27: Determine Δ<sub>r</sub>H° for the following reaction,2 NH<sub>3</sub>(g)+
Q39: What is the mass of H<sub>2</sub>SO<sub>4</sub> in
Q47: Nitric acid,HNO<sub>3</sub>,dissociates in water to form nitrate
Q48: Which of the given ions have the
Q58: The small,but important,energy differences between 3s,3p,and 3d
Q58: Which of the following elements is most
Q65: The figure below depicts a shooting target.The
Q87: Ammonia gas is synthesized according to the