What quantity,in moles,of hydrogen is consumed when 179.6 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ? H2(g) + 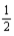 O2(g) → H2O(l) ; ΔrH° = -285.8 kJ/mol-rxn
O2(g) → H2O(l) ; ΔrH° = -285.8 kJ/mol-rxn
Definitions:
Organizational Innovation
The process of implementing new ideas, creating dynamic products, or improving services within an organization to enhance its processes and goals.
Coerce
To persuade (an unwilling person) to do something by using force or threats.
Role Modeling
The process of influencing others by exemplifying desirable behaviors and attitudes.
Self-Esteem
A person's personal assessment of their own value or importance.
Q6: What is the name of the element
Q21: How many grams of dioxygen are required
Q26: A dilute solution is prepared by transferring
Q39: The SI unit of temperature is the
Q46: What are the approximate F−Br−F bond angles
Q46: The following equation: A = ε ×
Q58: Which of the following elements is present
Q72: Which of the following elements would be
Q74: A flexible vessel is filled to a
Q81: A pressure of 1.00 atm has a