When 1 mole of Fe2O3(s) reacts with H2(g) to form Fe(s) and H2O(g) by the following reaction,98.8 kJ of energy is absorbed. Fe2O3(s) + 3 H2(g) → 2 Fe(s) + 3 H2O(g) 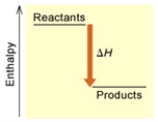
 (A)
(A)
(B)
Is the reaction endothermic or exothermic,and which of the enthalpy diagrams above
Represents this reaction?
Definitions:
Klinefelter's Syndrome
A genetic condition in males characterized by an extra X chromosome, leading to symptoms like low testosterone levels, reduced muscle mass, and infertility.
Chromosomal Analysis
A laboratory test that examines chromosomes in a sample of cells to identify genetic diseases or abnormalities.
Semen Analysis
A laboratory test of semen to assess its volume, sperm count, motility, and morphology, used in the evaluation of male fertility.
Testicular Biopsy
A medical procedure involving the removal of a small piece of tissue from one or both testicles for examination to diagnose infertility or detect cancer.
Q2: A gas sample is held at constant
Q4: In valence bond theory,each sigma bond in
Q14: Write a balanced chemical equation for the
Q23: What is the final volume of nitrogen
Q38: A bomb calorimeter has a heat capacity
Q38: The products of the complete combustion of
Q47: What is the molecular geometry around carbon
Q49: _ acid is produced in a larger
Q65: Place the following gases in order of
Q65: The figure below depicts a shooting target.The